Leading With Authenticity In High-Stakes Environments
- Led 20+ global submissions
- Drove SmPC harmonization across 24 regions
- Reduced regulatory review timelines by 50%
- Streamlined document preparation processes and increased submission quality across multiple regulatory and medical functions





Career Highlights
Strategic pharmaceutical executive with 15+ years of experience driving drug safety, regulatory affairs strategy, and compliance across global organizations.
Core Skills
Cross-functional team leader
Grace under pressure
Innovative people leader
Relationship builder
Spartan Competitor | Public Servant | PharmD
- Data-Driven Strategic Regulatory document preparation
- Uniquely equipped to align medical strategies with enterprise-wide priorities
- Foundational research experience
- 15+ years of pharma industry experience
- Boosted regulatory review efficiency by reducing rework and queries by 15%
Industries
Life sciences
Pharmaceutical
Biotech

Task/Activity:
Key Actions Taken:
- Issued technology stipends for employees with personal computers
- Produced and distributed how-to videos for OpenVPN setup and Microsoft Teams usage
- Provided one-on-one phone support for employees needing additional help
- Maintained operations across customer service, purchasing, and logistics
Case Study
Community Engagement
- In a previous role at a community pharmacy, I encountered a patient who was upset about a medication change that affected their chronic condition. I listened to their concerns and validated their feelings.
- I explained the rationale behind the change, ensuring they understood the benefits and potential side effects. I also coordinated with their HCP to discuss alternative options. The patient left feeling reassured and grateful for the personalized attention, reinforcing the importance of effective communication in pharmacy practice.
Results:
- Zero disruption in daily business operations
- Rapid upskilling of staff in remote collaboration tools
- Hundreds of thousands saved daily by avoiding downtime
- Strengthened company resilience and digital adaptability

Task/Activity:
Key Actions Taken:
- Wrote SQL queries to extract the requested data
- Double-checked work after submission and identified a flaw: an extra space in the LIKE clause
- Revised the query, validated results, and ensured data accuracy
- Proactively alerted my manager with an after-hours email
- Scheduled a morning meeting to take responsibility and explain the fix
- Requested to deliver the corrected data personally to the VP
Case Study
EU - Submissions
- I led a project involving the introduction of a new pharmaceutical product in the EU, which required navigating the stringent regulations of multiple member states.
- I coordinated with our cross-functional team, including legal and compliance, to ensure all documentation met local requirements while also advocating for expedited review processes. As a result, we successfully launched the product three months ahead of schedule, enhancing our market position significantly.
Results:
- Issue was resolved before the incorrect data was shared
- Built trust and credibility with both my manager and the VP
- Reinforced a habit of meticulous review and accountability that has carried through my career

Task/Activity:
Key Actions Taken:
- Researched and compiled a list of viable system replacements
- Encouraged the operations team to define their workflow and system requirements
- Created a draft process flowchart to initiate progress
- Led working sessions to collaboratively refine documentation
- Trained the operations team on documenting processes and gathering requirements
Case Study
Health Canada - Submissions
- In a previous role, I was responsible for preparing and submitting NDS applications to Health Canada. I followed a structured approach that included compiling clinical trial data, ensuring compliance with regulatory guidelines, and coordinating with cross-functional teams.
- One challenge was addressing unexpected feedback from the agency, but I organized a rapid response team to provide the necessary data, which ultimately led to a successful approval.
Results:
- Completed a full workflow and requirements specification
- Enabled timely selection of a new shipping system
- Empowered the operations team with lasting process analysis and documentation skills

Task/Activity:
Key Actions Taken:
- Analyzed the proposed workflow and identified inefficiencies
- Documented an optimized workflow focused on clarity and automation
- Presented a revised solution with visual flow diagrams in Jira
- Collaborated with the Purchasing Manager to review and refine the approach
Case Study
FDA - Submissions
- At my previous job, we needed to launch a new medication while navigating complex FDA regulations. I led the team in mapping out all regulatory requirements and coordinated with the development and marketing teams to ensure compliance.
- We faced a tight deadline but successfully submitted our application on time. As a result, we received approval within three months, allowing us to launch the product ahead of schedule, which increased our market share by 15%.
Results:
- Implemented a streamlined Jira workflow that reduced unnecessary notifications
- Improved task clarity and accountability across multiple departments
- Increased operational efficiency in the product launch process

Task/Activity:
My mandate would be to develop and execute a comprehensive U.S. medical strategy to support product differentiation, stakeholder engagement, and launch readiness within a tight window before the anticipated FDA approval.
- I would lead the creation of a Scientific Communication Platform (SCP) to harmonize core data messaging, which would inform all medical content, publications, and external engagements.
- A trained team of field MSLs would be needed to cover priority academic centers and community networks, to align their plans to our stakeholder mapping and insight objectives.
- I would design and execute an advisory board series with national KOLs to refine our evidence generation priorities and shape post-marketing study plans.
- Partnering with regulatory, HEOR, and commercial leads to finalize the integrated evidence generation plan, I would secure internal buy-in and global alignment.
I would also initiate congress strategy planning 6 months in advance to ensure high-impact visibility at ASCO and ESMO through symposia, scientific booth content, and coordinated KOL engagements.
Case Study
Medical Affairs - Launch Planning
Situation:
If I was tasked with preparing for the U.S. launch of a first-in-class oncology asset and our clinical data was promising but not widely differentiated, and internal alignment across cross-functional partners was fragmented, that would present as a problem.
Results:
- Achieve FDA approval on schedule and launch with a fully aligned and trained medical affairs team in place.
- 95% of targeted academic centers engaged within the first 60 days post-approval.
- Three investigator-initiated studies (IIS) initiated within the first quarter post-launch.
- Post-launch surveys indicating strong differentiation of our mechanism of action among prescribers, attributed to consistent scientific messaging.
- The SCP would become the foundation for all global medical communication and be adopted by EU and APAC affiliates.
Testimonials
What People Say
“She has this knack for ensuring that everyone is aligned and working efficiently towards shared objectives….. Her meticulous approach to reviewing and approving promotional content and safety data has been pivotal to both pre- and post-marketing activities.”
“Monique is detailed oriented with excellent knowledge of FDA, and EMEA regulations, and ICH, CIOMS guidances on pharmacovigilance. She produces quality of work and is outstanding at time management. Monique has more than five years of experience in pharmacovigilance. She has excellent communication skills and is very hard working with pleasant personality.”
“Monique is a passionate and visionary leader whose dedication to advancing health equity and elevating diverse voices in the biopharmaceutical industry is truly inspiring. Her career, community engagement, and commitment to health equity make Monique not only an exceptional professional but a true change-maker. She embodies the spirit of transformational leadership and collaboration that organizations and communities need to thrive.”
“Few people have the opportunity to work with a person like Linda. She is clever, creative and problem solver. As a program developer, Linda can quickly understand the needs of the end user and develop solutions that are above expectations. As a manager, I was able to witness how she kept an effective and efficient team. I highly recommend her.”
Scientific Journal Articles
Catalytic reduction of pertechnetate (99TcO4-) in simulated alkaline nuclear wastes
Novel ZnX2 -Modulated Pd/C and Pt/C Catalysts for Chemoselective Hydrogenation and Hydrogenolysis of Halogen-Substituted Nitroarenes, Alkenes, Benzyl Ethers, and Aromatic Ketones
Synthesis 2003(11): 1657-1660
DOI: 10.1055/s-2003-40878
Medical Articles
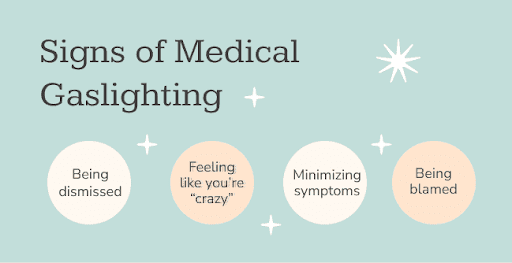
Medical Gaslighting - By Monique Richards
Data shows women, people of color affected most by ‘medical gaslighting’ (ABC News, 2022)
Steel Magnolia Enterprises
- Preparing corporate, US and EU labeling (prescribing information & patient information) documents
- Drug Utilization Review
Have you ever wondered, “Why am I taking this medication?”
It is very important for you to advocate for yourself (or your loved ones) when visiting healthcare providers. The days of blindly trusting the prescriber to handle your health are over.
You are your own best advocate. Let me know if you need me to provide a personal drug utilization review for you!
